Ideal for emerging and early-stage organisations.
Make your labelling and artwork process compliant from day 1
Standardised Industry Best Practice Workflow, Validated System, Speedy Deployment
The Life Science Industry's complex labelling demands are met by Perigord's GLAMS Q, a tailored system ensuring regulatory compliance and operational efficiency. With intuitive tools and streamlined workflows, it replaces outdated paper-based methods. Backed by over a decade of expertise, Perigord supports companies in their growth and market endeavors.
Validated streamlined processes: The core of GLAMS Q
By integrating industry standards and rigorous validation protocols, our software solution ensures unmatched compliance and efficiency for the Life Science sector.
The Life Science Industry's labeling and artwork processes are intricate due to strict regulations. Errors can lead to substantial financial setbacks, making traditional solutions like Excel outdated. To address this, we introduced GLAMS Q – a specialized Artwork Management System designed for the regulated landscape of this industry. Right from its inception, GLAMS Q incorporates validated and efficient workflows, ensuring seamless compliance and enhanced productivity. It allows users to streamline tasks, simplify proofing, and clarify approval stages, minimizing the inefficiencies of traditional trackers. With over a decade of engagement with Life Science entities, Perigord provides the expertise to facilitate business growth and penetrate new markets.

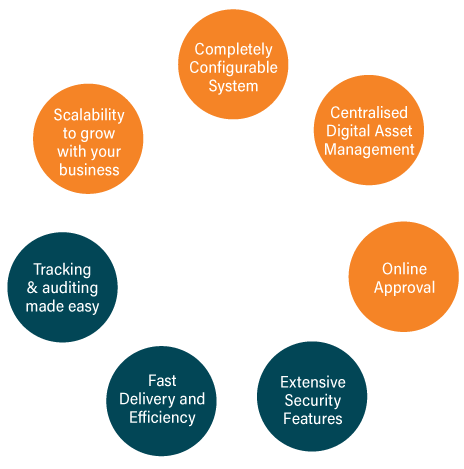
Achieve Better Results
Fully Validated Solution
GLAMS Q is a fully validated system and is the only artwork management system developed specifically for Life Sciences. It has been fully validated and designed for a highly regulated environment by an industry expert team. GLAMS Q doesn’t compromise, and provides the best, and most secure platform for artwork management.
Full Suite Of Proofing Tools
No more human errors! No more limitations in managing the proofreading of packaging. Make rejections post-approval a thing of the past. Carry out all graphics and text comparisons in one place with GLAMS Q, with its intuitive and powerful comparison tools. Annotate directly onto your artworks and collaborate with others to identify and eliminate errors completely.
Rapid deployment
GLAMS Q is ready when you are, with no lengthy install procedures or complex system requirements. Rapid deployment means that you can start improving your processes and KPIs immediately.
Speed-Up Your Approval Process
Save time through quick task assignment, easy to use proofing tools, best practice workflows and clear approval stages. Reduce time and wasted effort spent on tracking sign-off sheets, trawling through emails, searching for master files and interpreting clunky Excel based trackers. Enhance your team’s ability to do even more with GLAMS Q, while benefiting from time and cost efficiencies.
Prevent stockouts
With GLAMS Q, our end-to-end digital workflow allows you to intelligently search and quickly distribute your digital artwork assets. This will ensure that any stock outs due to artwork issues will be eliminated.
Pre-Configured System
GLAMS Q comes fully pre-configured with an industry best practice workflow. You are equipped from Day 1 with everything you need from an artwork management system. As with GLAMS Enterprise, you will be able to progress quickly to this package, when ready, at the click of a button.
Find out more about
reducing your artwork
proof cycle by 70%
We help Life Science companies design, manage and deliver packaging artwork, marcoms and digital assets
-

Packaging Artwork & Labelling Services
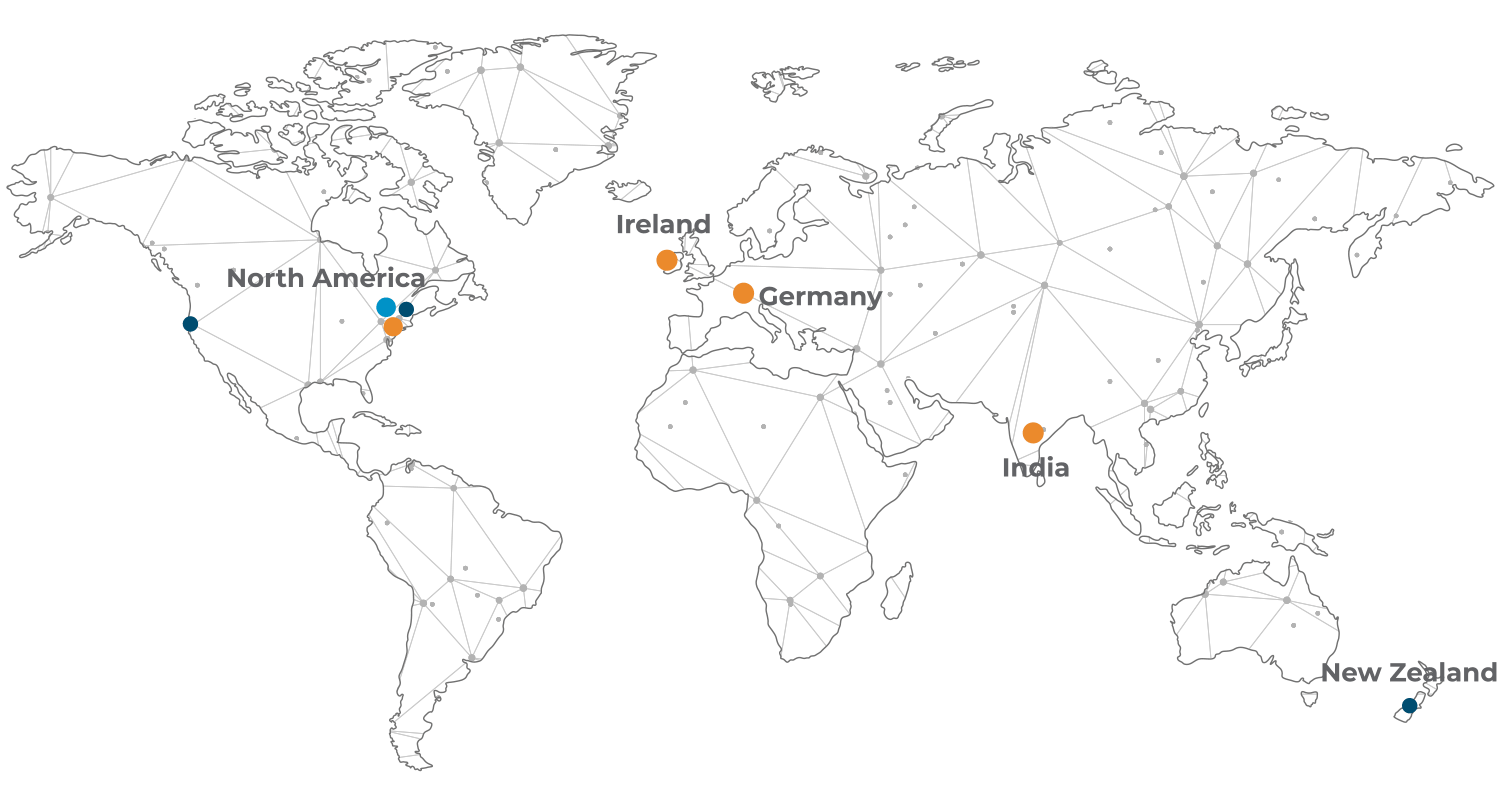
We provide global leadership and best-in-class customer engagement
in the provision of packaging artwork and labelling services for the Life Science industry.
Read more -

Strategic Consultancy
Our consulting team provides strategic advice on supply chain,
packaging/labelling, regulatory, quality, and marketing communications.
Button -

Creative & Digital Services
Our team delivers best-in-class creativity for the Life Science industry.
Helping you to visually communicate effectively through all channels.
Button -

Strategic Outsourcing (BPS)
With a highly competitive and regulated marketplace, it is becoming
the norm within the Life Sciences industry to outsource specialised tasks.
Button -

Managed Services
We manage complex supplier relationships taking full end-to-end
responsibility for the just-in-time delivery that help bring your products to market.
Button -

Software Solutions
Our purpose-built platform, GLAMS, is the world’s only workflow
management system designed specifically for the Life Science industry.
Button
Quality Guaranteed
About us
Latest News