GLAMS ePI
Enabling the shift to Electronic Product Information (ePI) – with expert services and adaptable technology solutions.
Supporting Life Science companies to meet the different regulatory authority requirements for ePI
In an evolving regulatory and digital landscape, GLAMS ePI supports Life Science companies in transitioning to structured electronic product information. From regional mandate readiness to automated conversion, our services and platform ensure accurate, compliant, and accessible ePI content, optimising both internal workflows and patient engagement.
Why ePI Matters
ePI enables structured, digital formats for healthcare product information, enhancing readability, accessibility, and regulatory compliance. It supports global trends toward digital transformation, enabling real-time updates and consistent content delivery across all platforms.
Global initiatives such as EMA’s ePI programme, the FDA’s Patient Medication Information rule, and Jordan’s JFDA mandate are accelerating adoption of standardised formats like XML HL7 FHIR. GLAMS ePI is designed to allow you to adapt to these different requirements.

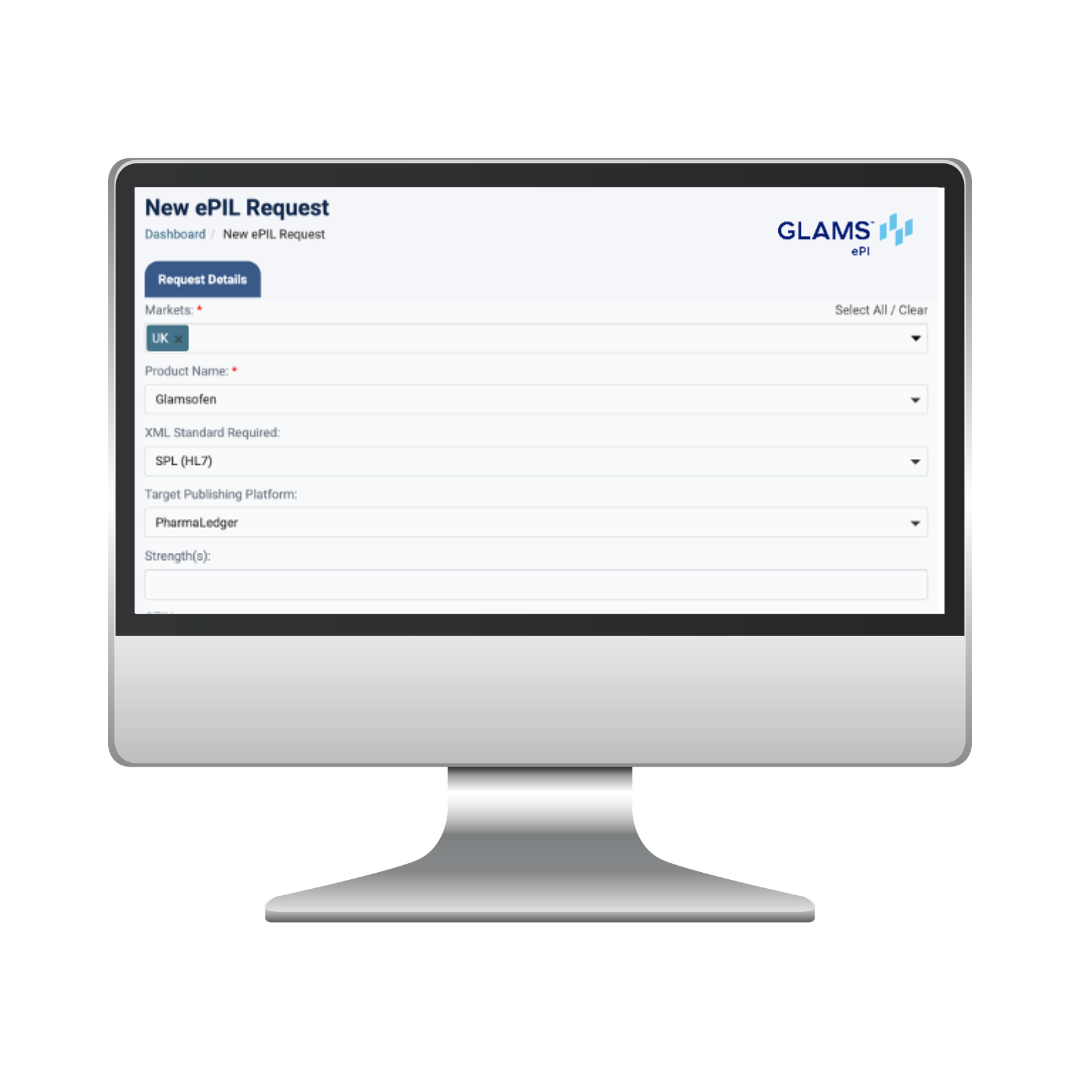
GLAMS ePI at a Glance
Our ePI solution combines expert consulting, conversion services, workflow automation, and integration capabilities to support your entire electronic product information journey:
ePI Readiness Consulting
Assess organisational preparedness and align with regional regulations (e.g., EMA, JFDA, PharmaLedger).
Conversion Services
From Word or PDF to XML (HL7 FHIR, PLA-compliant), including metadata generation and review tools.
Workflow & Automation
Automate content transformation, approval, and submission processes with scalable, structured solutions.
Technology Integration
Deploy GLAMS ePI as a standalone or integrated system to manage structured content and support digital dissemination.
Built for Life Sciences
GLAMS ePI is tailored to the needs of pharmaceutical and healthcare companies. Our software supports:
- Semi-structured content (both fixed and free-text elements)
- Multi-language outputs (e.g., Arabic, French, English)
- QR code integration
- Compatibility with structured submission standards and regulatory platforms
Whether you are just beginning your ePI journey or looking to scale existing capabilities, GLAMS ePI enables confident compliance and smoother digital transition.